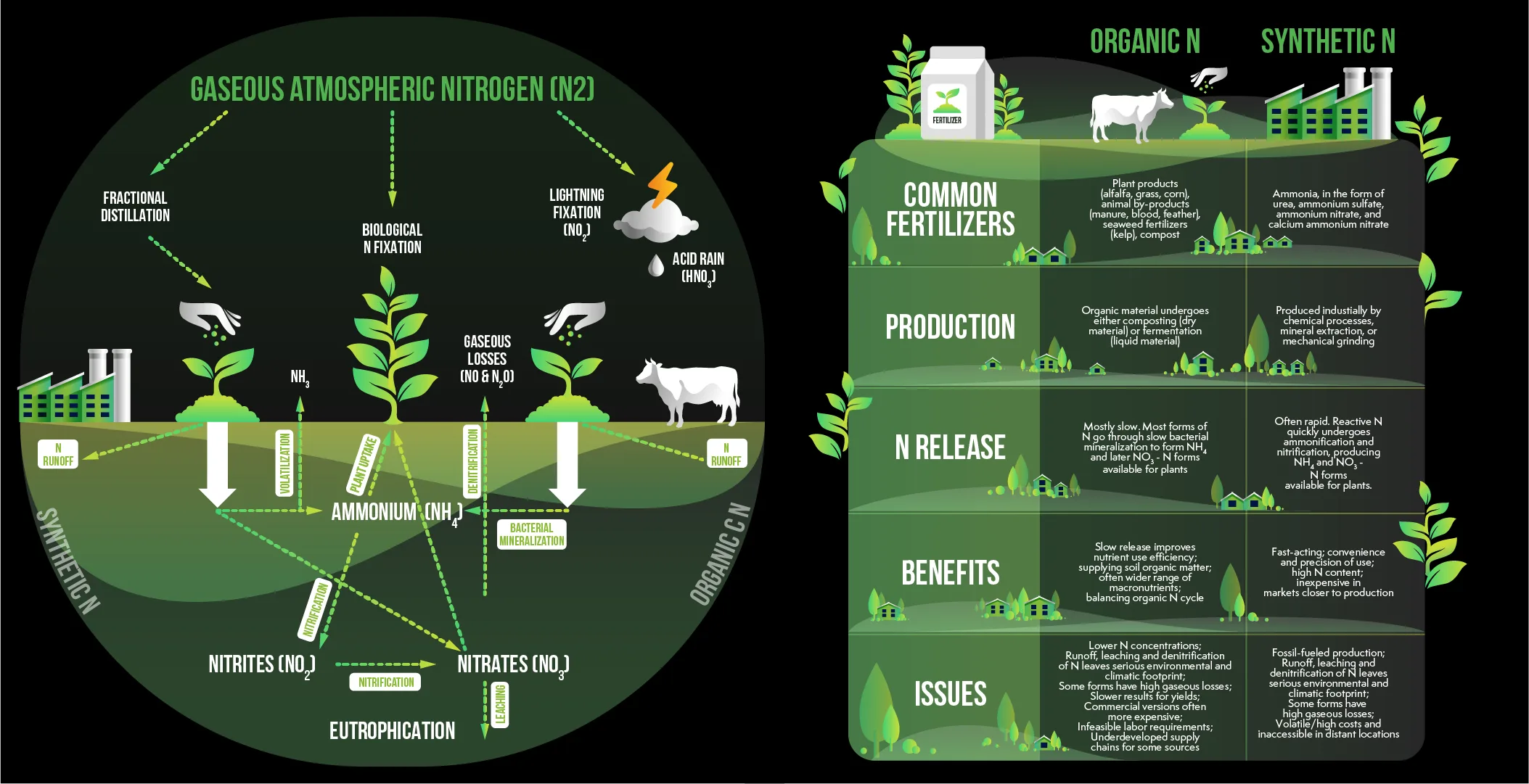
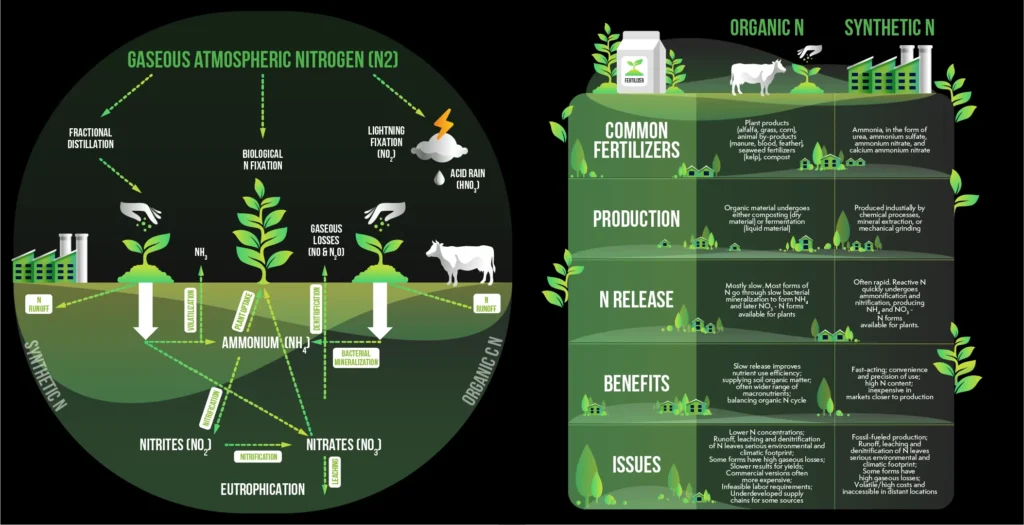
Nitrogen is at the heart of living things. It is the fundamental component that comprises DNA RNA and proteins which makes it vital component of every living thing. However despite comprising 78% of the atmosphere the vast amount of dioxigen (N2) is essentially unaccessible for the majority of living things.
Two nitrogen atoms of N2 are joined by an extremely powerful triple bond. It is which is one of the strongest bonds found in the world of. For N2 to be used by animals and plants the bond has to be broken in order for the nitrogen to be “fixed”..
or converted into an ammonia like reactive form (NH3). In the last century humans have relied on one process that has revolutionized the world that can accomplish this at large scales: the Haber Bosch procedure.
The Haber Bosch process is perhaps one of the biggest industrial innovations that was invented in the twentieth century. It is combination of atmospheric nitrogen and hydrogen under extreme temperatures (150 250 bar) as well as high temperatures (400 500degC) the process creates the ammonia which is the basis for nitrogen fertilizers.
Its helped us supply world population which has quadrupled in the past 100 years. But this marvel of modern chemistry is at the cost of huge environmental burden.
It is an extremely energy intensive process that consumes up to 2 1 percent of the planets energy sources. It is also completely dependent on hydrogen that comes from fossil fuels. This makes it accountable for nearly 1percent of the worlds carbon dioxide emission.
In 2025 at crucial point in our battle to stop climate change and environmental destruction the need to reinvent this essential chemical process has never been more crucial. It has led to the global search to find Green Nitrogen Fixation.
This comprehensive guide will take you through the intriguing and fast growing sector that is Green Nitrogen Fixation wide range of new techniques that are promising to create ammonia in sustainable manner.
Well explore the most cutting edge research and technology in electrochemical photochemical and plasma based approaches that are designed to unravel nitrogens triple bond with renewable energy instead of fossil fuels. This will pave the way for renewable energy and agricultural future.
The quest for Green Nitrogen Fixation is not simply scholarly exercise its crucial task to re invent one of the foundations of the modern world.
The problem with Haber Bosch The Reasons We Should Need Green Nitrogen Fixation
In order to appreciate the revolutionary nature that is Green Nitrogen Fixation we need to understand the deep history and deep seated issues of the Haber Bosch procedure. It was developed in the first half of 1900 in the early 1900s by Fritz Haber and Carl Bosch this process proved to be major scientific accomplishment. It was able to solve major problem that was threatening the world at the time which was the shortage of natural fertilizers such as Guano which threatened to constrain the production of food in the world.
The Heavy Footprint of Chemical Behemoth
The Haber Bosch process is run in mega scale centralized. Large chemical facilities typically situated near natural gas create ammonia which is transferred across the globe. The model is characterized by two major weaknesses in an environmentally sustainable future.
- Massive energy consumption and carbon emission: The process needs extreme conditions to break through the difficult NN triple bond. The conditions for this are met using natural gas (methane) in order to create the required heat and in addition to produce hydrogen gas that is required by steam methane reforming. Reliance of fossil fuels renders industrial ammonia production an enormous emitter of greenhouse gases which rivals the emissions of entire countries for the carbon footprint.
- Centralized Production Model advantages of scale require that Haber Bosch production facilities must be massive. Centralized models lead to extensive and intricate supply chains high volatility in prices and absence of resilience. The farmers in the remote areas typically face expensive transportation expenses as well as supply disruptions.
The Environmental Cascade of Over Fertilization
The great success of Haber Bosch has triggered new environment related crisis. The availability of inexpensive synthetic fertilizers has led to over application across the globe. The majority of this nitrogen isnt absorbed by plants and is instead pumped through rivers and streams. It can result in:
- Eutrophication: Excess nitrogen in the atmosphere causes massive blooms of algae that occur in lakes rivers and even oceans. If these algae die and break down and consume oxygen dissolved in the water resulting in huge “dead zones” where fish and aquatic animals cant survive.
- The emission of nitrogenous oxides from soil microbes have the ability to transform excess fertilizer into the gas nitrous oxide (N2O) which is powerful greenhouse gas that has an estimated warming capacity of 300 times the amount of carbon dioxide in one hundred year time.
The serious environmental effects of this underscore the need for urgent fundamental shift. It is time to find better way to make ammonia which does not only have clean environment and safe but also allows for greater decentralization and more accurate implementation. That is the main goal for Green Nitrogen Fixation. Development of sustainable Green Nitrogen Fixation methods is vital to decarbonize agriculture.
Electrochemical Nitrogen Fixation (Ammonia) is derived from water air and electricity
The most exciting ways to achieve Green Nitrogen Fixation is the electrochemical nitrogen reduction reaction (NRR). Its concept is simple using renewable energy to break up atmospheric nitrogen and water molecules and then combine with ammonia at temperatures of ambient temperature and pressure. The process is typically carried out using device that resembles the fuel cell or electrolyzer can completely transform the process of producing ammonia.
How It Works: The Principles of NRR
The electrochemical NRR cells typically is composed of two electrodes buried inside an electrolyte.
- The Cathode (The “Action” Site) The cathode is the place where the magic takes place. The gas of nitrogen from the air is pumped into the cathode. By using an specialized catalyst and then the use of an electric voltage which causes reduction in the nitrogen molecules it is diminished. It takes protons (hydrogen ions and H+) in the electrolyte (which result from splitting water molecules) as well as electrons from the outside circuits to create ammonia (NH3). The general reaction is N2+6H++6e 2NH3.
- It is also known as the anode (The “Supporting” Site) at the anode the oxidation process takes place in order to finish the circuit. It is usually an oxygen evolution reaction (OER) that occurs when water splits to create protons oxygen gas and electrons.
If the energy that drives this process is generated by renewable sources such as wind or solar energy and the ammonia that is produced is non carbon. This method is the foundation for Green Nitrogen Fixation.
The Catalyst Challenge: The Holy Grail of NRR
The main obstacle for Electrochemical NRR is finding good catalyst to be used for the cathode. This NN triple bond well known to be difficult to break and the catalyst has to be able to do it efficiently and without spending energy in unwanted reactions. The most important rival reaction is the reaction of hydrogen evolution (HER)
in which electrons and protons are combined to create the gas hydrogen (H2) rather than ammonia. Because HER is much simpler to trigger than NRR many catalysts generate lots of hydrogen and less ammonia.
The search for the ideal NRR catalyst will be the main area of material science research until 2025. Scientists are examining wide range of different materials. These include:
- Noble Metals: Ruthenium as well as the rhodium metals have demonstrated promise however they arent plentiful and costly for use on massive scale.
- Transition Metal Oxides Nitrides and Sulfides Transition Metal Oxides Nitrides and Sulfides have increased in abundance and are being engineered on the nanoscale to make high activity catalytic sites.
- Single Atom Catalysts: The revolutionary technique involves anchoring individual metallic atoms (like molybdenum iron) to conductive substrates. This improves the efficiency of every catalytic atom. It is the primary aspect of future research in Green Nitrogen Fixation technologies.
The Promise of Decentralization
The power behind electrochemical Green Nitrogen Fixation lies in the potential to decentralize. Instead of massive centralized facilities you can imagine smaller mobile “ammonia synthesizers” powered by local solar panels or wind turbine.
A small or even farm village could make the fertilizer itself on site as well as on demand. distributed system could reduce transport costs decrease the loss of energy and create an enduring and fair food system. It is the ideal concept for this kind of Green Nitrogen Fixation.
The Photochemical Fixation of Nitrogen: Making Ammonia by exposing it to sunlight
The concept that Green Nitrogen Fixation step beyond photochemical NRR seeks to harness the sunlights energy directly to trigger the synthesis of ammonia. This mimics the basic process of photosynthesis. This technique eliminates the requirement to convert sunlight energy into electricity giving quicker and more efficient route.
The Mechanism: Solar Powered Catalyst
In photochemical process that is specialized the material known as photocatalyst is able to absorb the photons (particles made of light). The absorption of energy stimulates electrons inside the catalyst making them accessible to take part within chemical reactions.
It is the same process as the electrochemical method: photocatalyst utilizes the power of the sun to split the water molecules and to stimulate nitrogen molecules attracted to its surfaces. Protons and electrons that are energized can then be used to convert the nitrogen level to ammonia. The whole process is fueled by light. It is an extremely elegant method of Green Nitrogen Fixation.
Designing the Perfect Photocatalyst
Photocatalysiss challenges are the same as those encountered in electrocatalysis. They are based on designing high reliable and durable catalyst. An ideal NRR photocatalyst needs to:
- The ability to absorb Broad Spectrum of Sunlight: majority of catalysts can only absorb the high energy UV light that is only tiny portion of the spectrum. Its the aim of creating catalysts that also absorb the abundance of visible sunlight.
- efficiently separate and use charges When photon has been absorbent the resultant excited electron as well as its “hole” it leaves behind need to be separated from the rest and directed towards the sites of reaction prior to recombine and consume the energy.
- You can have high selectivity NRR: Similar to in electrocatalysis. The catalyst should favor the more difficult NRR reaction over the simpler hydrogen evolution reaction.
Researchers are developing intricate nanomaterials that often combine semiconductors (like titanium dioxide and Cadmium sulfide) together with co catalysts (like gold or platinum nanoparticles) to satisfy these stringent specifications. Surface modification and defect engineering are two key methods being employed in the creation of materials specifically designed for this particular form of Green Nitrogen Fixation.
Although the photochemical NRR is still at different phase of development that its electrochemical counterpart the potential for it to develop an easy single step procedure for the synthesis of solar ammonia is what creates an thrilling new frontier within the area of Green Nitrogen Fixation.
Plasma Assisted Nitrogen Fixation: Electrifying the Air
A different method of electrifying Green Nitrogen Fixation uses plasma which is the fourth state of matter. Plasma is an inorganic gas with ions that contains mixture of electrons ions as well as neutrons. When we apply powerful electrical field to the air (which comprises mainly oxygen and nitrogen) it is possible to create the plasma which has the energy to break through the NN triple bond.
How Plasma Catalysis Works
In plasma reactor the electrical discharge (like the tiny and targeted lightning flash) is pushed by stream of air or through mixture of nitrogen and hydrogen.
- Vibrational Excitation : The high energy electrons of the plasma clash with nitrogen molecules. Instead of instantly breaking the bonds the collisions transmit energy back to the molecules which causes it to oscillate.
- The Stepwise Bond Breaking: With each collision the molecule is ever more “vibrationally excited.” The process gradually weakens the triple bond until eventually it is broken which results in high reactivity nitrogen Atoms.
- Reaction that results in Ammonia or NOx The reactive nitrogen atoms may then react with hydrogen forming ammonia (NH3) or when oxygen is present together with oxygen to produce the nitrogen oxides (NOx). The NOx are readily converted into nitric acids which is different important nitrogen based chemical as well as fertilizer.
The main benefit of using plasma for Green Nitrogen Fixation is that it can be switched up and off in matter of seconds and is ideally suited for use with renewable sources of energy that are intermittent such as solar or wind. If the sun shines or wind blows it can be turned on to create fertilizer.
The Non Thermal Plasma Advantage
Researchers are particularly interested in “non thermal” or “cold” plasmas. These systems are where the electrons are extremely energized and the gas itself remains close to room temperature. This permits the rapid break of the NN bond without the waste of huge quantities of energy to heat the entire gas stream one of the major disadvantages of the Haber Bosch method.
The combination of plasma and catalyst (plasma catalysis) could further increase the efficacy and effectiveness of the process bringing the technology towards commercialization. This is an effective and quickly advancing pillar of Green Nitrogen Fixation.
Biology and Biomimetic Methods of Learning: From Nature
Nature naturally has done Green Nitrogen Fixation for billions of years. Some archaea and bacteria called diazotrophs contain fantastic enzyme dubbed nitrogenase. It is the natural answer to tearing the triple bond. And it accomplishes this feat with amazing performance at any temperature or pressure.
Nitrogenase: Natures Master Catalyst
The metalloenzyme Nitrogenase is an intricate metalloenzy which utilizes highly sophisticated cofactor of iron and molybdenum (FeMoco) in its active site to bond and decrease nitrogen. Understanding the complex mechanism behind nitrogenase can provide plan for the ideal Green Nitrogen Fixation process. Even though directly utilizing the enzyme or bacteria in industrial environments can be challenging because of their oxygen sensitivity and lower production levels The principles they apply can be used to create new line of investigation.
Biomimetic Catalysis: Mimicking the Master
Biomimetic chemistry is method of creating chemical catalysts which mimic the structure and functions of the active nitrogenase site. Scientists are creating complicated metal organic molecules which can mimic the mechanism by which nitrogenase bonds to the molecule N2 and controls the sequential addition of protons and electrons.
The aim is to create the first molecular catalyst that is synthetic that can be employed for photochemical or electrochemical cell. This catalyst could blend the superior effectiveness and selectivity of natural enzyme with the strength and capacity required by an industrial procedure. Although this is long term study goal the achievement in this field could be the final accomplishment of Green Nitrogen Fixation.
Enhanced Biological Systems
A different approach is to use genetic engineering to enhance the natural nitrogen fixation process of biological organisms. This can involve:
- Engineering Crops Changes to cereal crops such as corn and wheat to ensure that they are able to form symbiotic relations with nitrogen fixing bacteria like soybeans and legumes form symbiotic relationships. The crops would be able to make themselves fertilizers directly within their roots.
- Enhancing Soil Microbes by Developing improved strains of nitrogen fixing soil microbes that are more productive and resilient. These can be used in fields to act as an “biofertilizer.”
The biological options for Green Nitrogen Fixation aim to lower our dependency of fertilizers made from synthetic chemicals and create more sustainable and sustainable agriculture system.
The Road Ahead: Challenges and Future Outlook for Green Nitrogen Fixation in 2025
The way to create new world driven through Green Nitrogen Fixation can be extremely exciting however its not without obstacles. By 2025 this sector is the most active in research and development. However there are major hurdles that need to be get over.
The “Ammonia Contamination” Issue
One of the major contests that have dominated an electrochemical NRR community has been the question of contamination. Researchers have found that lot of the first highly positive reports on ammonia production resulted from contamination with nitrogen from laboratories the equipment and chemicals or the gas sources.
This led to the creation of rigorous standardized protocols for testing to assure that the ammonia observed is actually the result from Green Nitrogen Fixation and not the result of contamination.
Efficiency Rate and Selectivity
In order for the use of any Green Nitrogen Fixation technology to be viable commercially it has to meet three performance indicators that are essential to its success:
- The Faradaic efficiency (for electrochemistry) What proportion of electrical energy is used to create the final product (ammonia) against undesirable side products (hydrogen)?
- Production Rate What amount of ammonia could be created per unit of time and/or per square meter of the reactor or catalyst?
- Process Selectivity: Can it be controlled so that it only produces ammonia without producing any other nitrogen based products?
Currently there is no Green Nitrogen Fixation technology has been able to compete with the process of Haber Bosch across all of these areas simultaneously. This gap in performance has been the principal goal of ongoing study.
System Integration and Scaling
The transition from lab scale experiment into robust actual world application is an enormous engineering problem. This requires designing reliable reactors and devising effective strategies for segregating the produced ammonia from the gas stream as well as integrating all of the components with an energy source that can be variable.
Pilot projects and start ups are beginning to take on the crucial challenges of scaling. Successful scaling of Green Nitrogen Fixation systems is the next crucial stage.
A New Chemical Revolution for Sustainable Planet
In the 20th century it was in variety of ways built upon the foundations of ammonia from Haber Bosch. It was the main source of food for all mankind and allowed for an incredible growth in the population of humans.
The 21st century however is going to be determined by our capacity to repair the ecological damage caused by the past and create an environmentally sustainable society. Green Nitrogen Fixation lies at the heart of this change.
The variety of different technologies ranging from the electrochemistrys decentralized power as well as the beauty of photocatalysis to the pure potential of plasma as well as the science behind biology is part of an exciting new era in chemical science.
Although the road isnt over yet however there is clear sign of progress. Scientists across the globe are committed to finding catalysts and systems that are able to efficiently and economically transform nitrogen into ammonia by using the only renewable sources.
The goal of all is to envision world that our food and energy technology are not the main driver of global warming. The future will be circular economy of nitrogen that is where Green Nitrogen Fixation technologies provide fertiliser that is clean for the agricultural sector and generate green ammonia for use for carbon neutral energy and fuel storage for energy.
Looking ahead to 2025 the development and eventually deployment the use of Green Nitrogen Fixation will be one of the strongest tools to restore the planet and sustain humanity into the future. Green Nitrogen Fixation holds promise. Green Nitrogen Fixation is the guarantee of healthier as well as more fair and more sustainable future.
- Digital Health Guide 2025: Navigating the Future of Wellness
- Picsart AI Photo Editor Video: The Ultimate Guide to Mastering Visual Content in 2025
- Green Nitrogen Fixation: The Ultimate Guide to Sustainable Future (2025)
- Flexible Electronics: The Ultimate Guide to Bendable Wearable Future in 2025
- Elastocalorics: The Ultimate Guide to the Future of Cooling in 2025